41 how are the labels in each period alike
Labeling, ranking, sorting, or sentence completion questions To deselect a label, tile, or term — To send them back to the list, press Esc. To place a selected label, tile, or term on a target ... To rank multiple tiles as equal, position them above, below, or on top of each other in the answer bin. The background color changes to a vertical band, indicating that the tiles share the same rank. The Periodic Table | Chemistry I | | Course Hero The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column. In the United States, the labels traditionally were numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common.
Drag each tile to the correct location on the image. The tiles are used ... Label each excerpt with the literary period it belongs to. romanticism realism 2 See answers Advertisement Advertisement annyksl annyksl Answer: 1-realism 2-Romanticism 3-romanticism 4-realism Explanation: The characteristics of realism are related to ability to demonstrate reality in the most believable way possible.
How are the labels in each period alike
The Periodic Table: Families and Periods - dummies Just like human families, members of the families (vertical columns) in the periodic table have similarities or similar properties. The families are labeled at the top of the columns in one of two ways: The older method uses Roman numerals and letters. Many chemists prefer and still use this method. The newer method uses the numbers 1 through 18. The Periodic Table and Energy-Level Models - Middle School Chemistry There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level. Tell students that the rows across on the periodic table are called periods. Period 1 Hydrogen Explain that hydrogen has 1 proton and 1 electron. The 1 electron is on the first energy level. Helium The modern periodic table - BBC Bitesize The modern periodic table arranges. elements. into a unique arrangement that can be used for reference. Metals are found on the left of the periodic table and non-metals on the right. The periodic ...
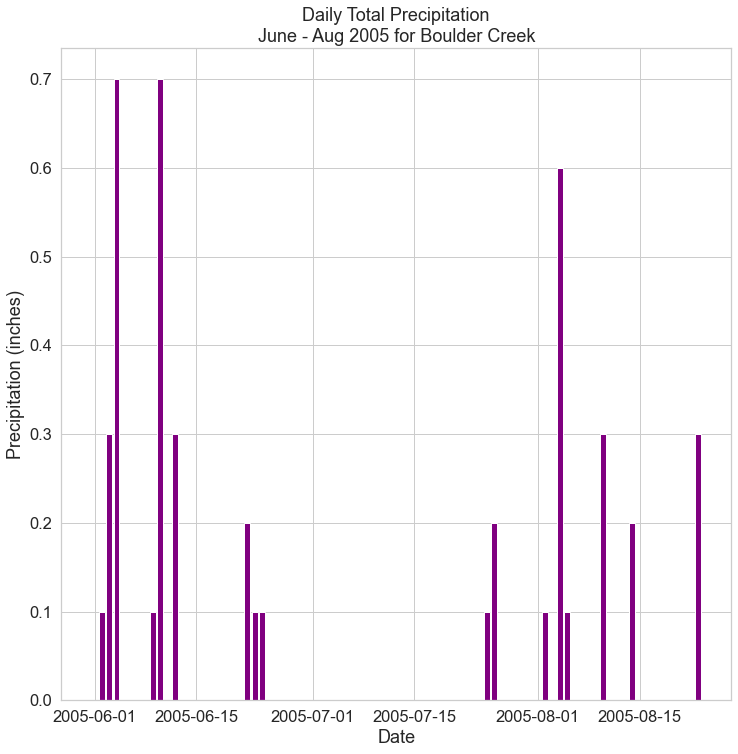
How are the labels in each period alike. Frederic C. Kaplan, Picture Maker - CCCART APPRECIATION assignment 2 ... Create a graphic timeline that is similar to the example segment shown below according to the instructions. ... use color markers to indicate the duration of each art historical period as listed below. Label each period and give its dates. Use the same color for each period and its sub-divisions. For example, if the Renaissance is blue, blue ... Modern Periodic Table: Periods and Groups - Course Hero A period is a horizontal row of the periodic table. There are seven periods in the periodic table, with each one beginning at the far left. A new period begins when a new principal energy level begins filling with electrons. Period 1 has only two elements (hydrogen and helium), while periods 2 and 3 have 8 elements. Tracking SARS-CoV-2 variants - World Health Organization All viruses, including SARS-CoV-2, the virus that causes COVID-19, change over time. Most changes have little to no impact on the virus' properties. However, some changes may affect the virus's properties, such as how easily it spreads, the associated disease severity, or the performance of vaccines, therapeutic medicines, diagnostic tools, or other public health and social measures. Line Graph (Line Chart) - Definition, Types, Sketch, Uses and Example Draw and label the scale on the vertical and horizontal axis. List each item and locate the points on the graph for both the lines. Connect the points with line segments separately of both the lines. Draw two line graphs within one chart. Example: See the graph below.
Characteristics of the Periods and Groups of the Periodic Table In a period, the atomic size decreases from left to right. The number of protons and electrons increases over time as the atomic number increases, therefore the extra electrons are added to the same shell. The electrons are drawn in closer to the nucleus due to the strong positive charge on the nucleus, and the atom's size shrinks. The Difference Between an Element Group and Period - ThoughtCo Groups and periods are two ways of categorizing elements in the periodic table. Periods are horizontal rows (across) the periodic table, while groups are vertical columns (down) the table. Atomic number increases as you move down a group or across a period. Element Groups Elements in a group share a common number of valence electrons. Periodic table - Wikipedia Overview. The periodic table is a 2-dimensional structured table. The elements are placed in table cells, in reading order of ascending atomic number.The table columns are called groups, the rows are called periods.The breaks at the end of each period occur according to a repetition (or periodicity) of physical and chemical properties of the elements. 5.1 The arrangement of the elements | The periodic table | Siyavula Another way to label the groups is using Roman numerals. A period is a horizontal row in the periodic table of the elements. The periods are labelled from top to bottom, starting with 1 and ending with 7. For each element on the periodic table we can give its period number and its group number.
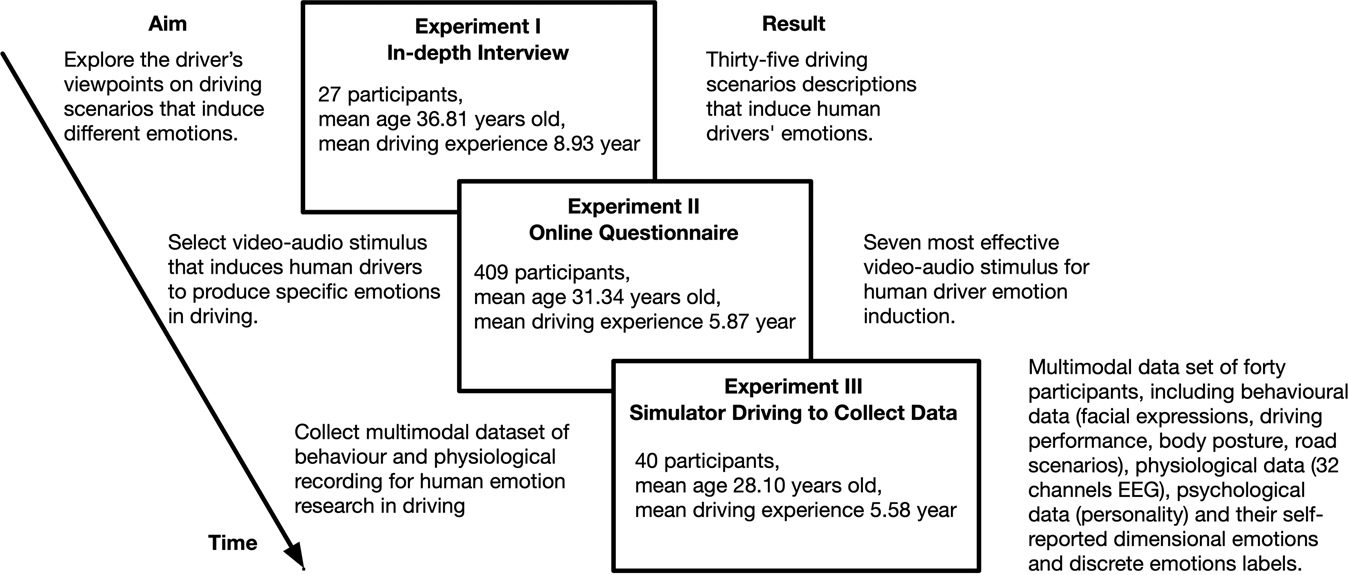
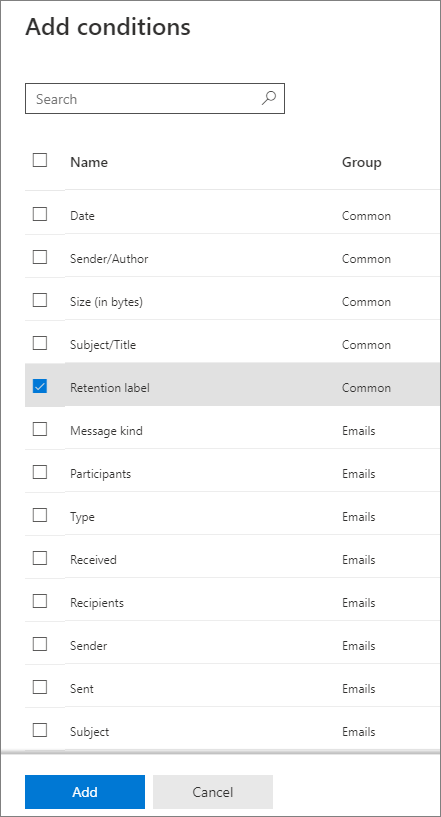
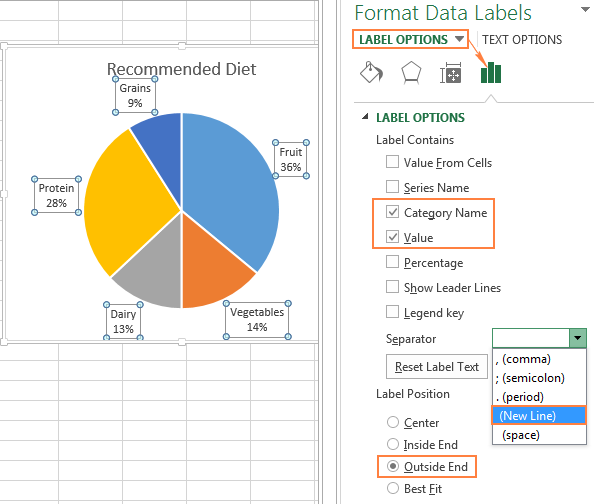
Reading the Periodic Table - California State University, Northridge Our current periodic table lists 109 elements. It is arranged according to the periodic law: Elements are arranged in order of increasing atomic number, their physical and chemical properties show a periodic pattern. Elements that have similar properties are arranged in groups or families - vertical columns The horizontal rows are called periods. Difference Between Periods and Groups Within a period, the elements share the outer shell (valence) electrons. For example, hydrogen and helium are in the same group and have one electron in the outer shell. Notice that the first period has only two elements. The second and the third periods have 8 elements each. Periods 4 and 5 each have 18 elements. Inquisitive 7 EXAM 2 Flashcards | Quizlet physiological records participants' own views of themselves self-report the number of times someone checks the clock in a waiting room observational measure someone's blood pressure physiological measure how depressed someone says they feel self-report measure the number of seconds someone spends smiling during an interaction observational measure Change the format of data labels in a chart To get there, after adding your data labels, select the data label to format, and then click Chart Elements > Data Labels > More Options. To go to the appropriate area, click one of the four icons ( Fill & Line, Effects, Size & Properties ( Layout & Properties in Outlook or Word), or Label Options) shown here.
Period (periodic table) - Wikipedia A period in the periodic table is a row of chemical elements.All elements in a row have the same number of electron shells.Each next element in a period has one more proton and is less metallic than its predecessor. Arranged this way, elements in the same group (column) have similar chemical and physical properties, reflecting the periodic law.For example, the halogens lie in the second-to ...
Regions of the Periodic Table - Mr. Bigler period: a row of the periodic table. Properties of the elements are periodic, meaning that they repeat after a specific interval. Elements in the same period have their highest energy electrons in the same principal energy level. group (family): a column of the periodic table. Elements in the same group have the same number of valence electrons ...
Properties of Periodic Table of Element Groups - ThoughtCo There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals. You'll find more specific groups, like transition metals, rare earths, alkali metals, alkaline earth, halogens, and noble gasses. Groups in the Periodic Table of Elements
Geologic Time Scale: Eons, Eras, and Periods - ThoughtCo Eons are divided into eras, which are further divided into periods, epochs, and ages. Geologic dating is extremely imprecise. For example, although the date listed for the beginning of the Ordovician period is 485 million years ago, it is actually 485.4 with an uncertainty (plus or minus) of 1.9 million years.
CHEM 1305: General Chemistry I—Lecture - Course Hero The elements are arranged in seven horizontal rows, called periods or series, and 18 vertical columns, called groups. Groups are labeled at the top of each column. In the United States, the labels traditionally were numerals with capital letters. However, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common.
Groups and Periods in the Periodic Table - breakingatom.com Periods are the rows of the periodic table that from left to right increase in order of mass Group 1 Group 1 elements are in the first group of the periodic table with similar properties of being soft and reacting violently with water Mass Mass is the amount of substance that can be measured in grams or kilograms Learn the Periodic Table!
Groups and periods - Groups and periods - GCSE Chemistry (Single ... - BBC there are only two elements in Period 1 (hydrogen and helium) The zig-zag line in this diagram separates the metals, on the left, from the non-metals, on the right. Hydrogen is a non-metal but it...
The modern periodic table - BBC Bitesize The modern periodic table arranges. elements. into a unique arrangement that can be used for reference. Metals are found on the left of the periodic table and non-metals on the right. The periodic ...
The Periodic Table and Energy-Level Models - Middle School Chemistry There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level. Tell students that the rows across on the periodic table are called periods. Period 1 Hydrogen Explain that hydrogen has 1 proton and 1 electron. The 1 electron is on the first energy level. Helium
The Periodic Table: Families and Periods - dummies Just like human families, members of the families (vertical columns) in the periodic table have similarities or similar properties. The families are labeled at the top of the columns in one of two ways: The older method uses Roman numerals and letters. Many chemists prefer and still use this method. The newer method uses the numbers 1 through 18.
![How is Brandy Created? [Infographic] | VinePair](https://vinepair.com/wp-content/uploads/2022/08/how-is-brandy-created-infographic-scaled.jpg)








/6-types-of-relationships-and-their-effect-on-your-life-5209431_V1-a0f57cea6a114b9cbfa1553c0142ec92.png)




![3 Types of Line Graph/Chart: + [Examples & Excel Tutorial]](https://storage.googleapis.com/fplsblog/1/2022/06/line-graph-features.png)




:max_bytes(150000):strip_icc()/Clipboard01-e492dc63bb794908b0262b0914b6d64c.jpg)

Post a Comment for "41 how are the labels in each period alike"